Precise Targeting
for Neurosurgery
RebrAIn aims to enhance patient outcomes in Parkinson’s Disease
and Essential Tremor treatments by combining
best-in-class supervised AI with clinically successful results.
RebrAIn is a spin-off of the University Hospital of Bordeaux and the Inria, based in the EU and the US, created by Professor Emmanuel Cuny, Neurosurgeon, and Professor Nejib Zemzemi, Researcher at Inria in Applied Mathematics, after 10 years of academic research and 2 theses.
RebrAIn’s service is a best-in-class, supervised AI-driven solution that streamlines complex, patient-specific neurosurgery by providing faster, simpler, and safer treatment through augmented neuromodulation planning.
Regulatory
CE marked,
FDA 510(k) cleared
Adoption
55 sites
worldwide
Indications
Parkinson’s disease (STN),
Essential tremor (VIM)
Volume
500+ targeting
operations performed
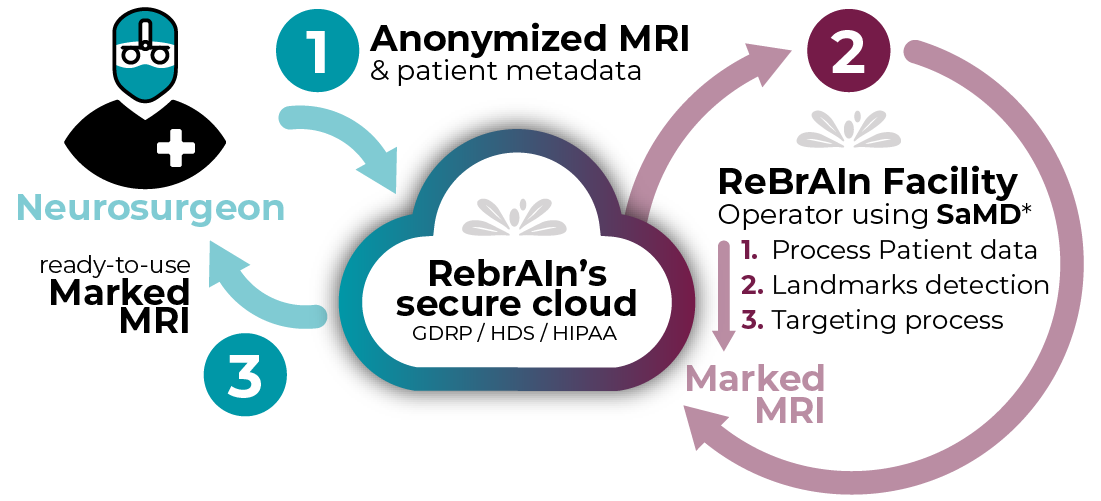
From T1 MRI upload to surgery planning,
RebrAIn services streamlines the pre-surgical planning process.
We have developed a targeting service that uses supervised AI algorithm & health data exchange platform to simplify and standardize neurosurgical procedures.
Our solution seamlessly integrate supervised AI-guided targeting into any workflow to streamline and standardize neurosurgical procedures.
* RebrAIn’ service leverages CE Marked and FDA 510(k) cleared Software as a Medical Device (SaMD), OptimMRI and OptimDBS.
With RebrAIn, we aim to enhance efficiency and peace of mind
for neurosurgeons and staff by providing millimetric MRI-guided
targeting of the STN or VIM based on a health data exchange platform.
Seamless integration
Surgery
under GA
Fast
workflow
Precise
surgery
Safe and efficient
Real Life impact
Testimony of a Parkinsonian patient treated on with the help of RebrAIn’ services
Testimony of an Essential Tremor patient treated on with the help of RebrAIn’ services
Real Life impact
Testimony of a Parkinsonian patient treated on with the help of RebrAIn’ services
Testimony of an Essential Tremor patient treated on with the help of RebrAIn’ services
Marked MRIs
worldwide
best-in-class
clinical data
Key anatomical
landmarks
Our latest public updates
RebrAIn Achieves MDR Certification for OptimMRI, Expanding Access to Precision Neurosurgery Across Europe
Already FDA 510(k) cleared in 2024, OptimMRI is an AI-powered surgical planning platform that supports Deep Brain Stimulation (DBS) and lesioning procedures (HIFU & Radiosurgery) targeting the STN and VIM regions. With MDR certification now secured, RebrAIn is...
RebrAIn joins the Charter for the Responsible Development of Neurotechnologies
RebrAIn is proud to announce its commitment to the Charter for the Responsible Development of Neurotechnologies, a collective initiative led by public and private stakeholders and supported by the French National Consultative Ethics (CCNE). This initiative is part of...
Challenges showcases RebrAIn’s AI-powered innovation in Parkinson’s surgery
Challenges underscores the significance of RebrAIn's technology in enhancing the precision and efficiency of deep brain stimulation (DBS) surgeries. By leveraging machine learning algorithms trained on extensive clinical data, RebrAIn's software assists neurosurgeons...