Science
RebrAIn service offers a precise targeting technology
for Deep Brain Stimulation (DBS) or lesioning**.
What is Deep brain stimulation (DBS)?
10% of patients with Parkinson’s Disease and Essential Tremor do not respond to pharmacological treatments, necessitating alternative methods.
Developed in France in the late 1980s by Prs. Benabid and Pollak, DBS is a neurosurgical technique designed specifically for these movement disorders. Often described as the brain’s pacemaker, DBS involves the delivery of electrical pulses to specific motor control areas.
This procedure requires millimetric targeting and implanting electrodes, with the standard of care being a very uncomfortable procedure performed with the patient awake, presenting a significant challenge for both neurosurgeons and patients.
To go further about DBS
Pioneers of Deep Brain Stimulation
Deep Brain Stimulation (DBS) was pioneered by Drs. Alim-Louis Benabid and Pierre Pollak in Grenoble, France, during the late 1980s. This innovative technique, utilizing electrical impulses to modulate brain activity, led to the development of a groundbreaking surgical method with electrode implants. The inaugural successful DBS operation in 1987 marked a pivotal moment in neurosurgery, providing significant tremor reduction in a Parkinson’s patient. This achievement positioned DBS as the ultimate therapy for patients not responding to conventional medications in the treatment of neurological disorders.
Technological Advances in DBS
The evolution of DBS has been characterized by advances in technology and a deeper understanding of brain targeting, essential for customizing treatment to individual patient needs. Innovations such as directional leads and adaptive DBS underscore the shift towards personalized care, allowing for precise brain targeting, potentially minimizing side effects and enhancing treatment effectiveness. Over the years, DBS has transitioned from a generic approach to a more refined, patient-specific strategy, paving he way to the modern era of DBS with improved targeting techniques.
Interdisciplinary Collaboration in DBS
The latest development in DBS is a testament to the collaboration across multiple disciplines, including neurosurgery, neurology, artificial intelligence, and biomedical engineering. This interdisciplinary approach has led to the precision targeting of brain areas for each patient. Pioneered by Prs. Emmanuel Cuny (University Hospital of Bordeaux) and Nejib Zemzemi (Inria), the method described below leverages cutting-edge technology and computational modeling to revolutionize personalized DBS targeting.
Supervised AI-Driven Precision in DBS
RebrAIn, a spin-off from the University Hospital of Bordeaux and the Inria* created by these pioneers, represents this innovative leap with its AI-driven technology that increases the precision of surgical interventions for Parkinson’s disease and essential tremor.
By analyzing clinical improvements post-operation and utilizing AI to pinpoint the most effective stimulation targets, RebrAIn aims to enhance surgical outcomes and standardize procedures. This approach not only promises to make surgery less traumatic but also seeks to extend global access to DBS therapy, potentially making DBS an even more efficacious treatment for Parkinson’s and other neurological conditions. Through personalization, RebrAIn aligns with the broader medical trend towards tailored therapies, ensuring patient outcomes are maximized.
Neurosurgery is a personalized treatment that requires a perfect mapping of the patient’s brain to target specific brain areas accurately and safely.
This precise position is complicated by the fact that the best electrode position is not visible under standard MRI, requiring more advanced methods to be applied.
The challenge for
80% of the centers
Complex, heterogeneous,
not reproducible, long

Intraoperative awakening of the patient required for micro-electrode recording (MER) & clinical evaluation
Traumatic, invasive, expensive, long.
Shortcomings that prevent access
Less than 3% of eligible patients undergo DBS treatment due to the complexity of accurately and safely targeting specific brain areas.
DBS treatments are highly personalized, increasing the complexity, risk, expenses, and time. This is often traumatic for patients, leading to inconsistent results due to imperfect brain mapping.
DBS procedures are expensive and lengthy, often traumatic for patients, with inconsistent results due to imperfect brain mapping.
Parkinson
|
Essential
|
| 10M patients |
50M patients |
| 1M eligible for surgery |
5M eligible for surgery |
| 30.000 patients treated |
30.000 patients treated |
| < 3 %of the eligible patients | < 0.6 %of the eligible patients |
RebrAIn' services offers a precise targeting technology for Deep Brain Stimulation (DBS) or lesioning**.
This is achieved through supervised AI algorithms and a health data exchange platform to identify areas of the brain to be treated. RebrAIn simplifies and standardizes DBS and cerebral lesioning**, while reducing risks and trauma.
** Work in progress
- Learns from patients with post-op clinical improvement
- Uses Artificial Intelligence to reproduce success
- Works with the simplest MRI sequences
- Provides marked MRI in the regions of interest
- Processes the MRI on the cloud
- Allows a secure exchange of health data in compliance with the GDPR
- Hosts data on HDS (Health Data Hosting) certified servers
Marked MRI
worldwide
best-in-class
clinical data
Key anatomical
landmarks
From facial recognition to brain recognition
Starting from a T1, 1.5 T MRI without injection, RebrAIn' services re-orients the MRI then recognizes 18 anatomical structures per side in a semi-automatic way.
After having “recognized” the brain as facial recognition systems recognize faces, it then applies the mathematical metamodel based on Artificial Intelligence which predicts the targets and marks them on the MRI.
A team of neurosurgical trained professionals are the ones responsible for verifying the anatomical points on the MRI. It is this marked MRI that is sent back to the healthcare centers to help in planning neurosurgical procedures.
Operational Benefits
Economic Efficiencies
Reduction of operational costs by reducing OR time and resources (i.e. MER), MRI scan time (no DTI needed), and length of hospital stay, ensuring higher profit margins.
Marketability
Our disruptive technology utilizing supervised AI technology elevates an organization’s prestige, making them more marketable to prospective surgeons and patients.
Expand Patient Base
The solution increases workflow efficiency, meaning more patients can be seen. It enhances operating room efficiency, enabling more surgeries and expanding patient care capabilities for neurostimulation patients.
For the healthcare provider, RebrAIn’ services enhances surgical efficiency, reduces procedure durations, and lowers patient risks, optimizing overall hospital operations.
Workflow
|
Financial
|
| One less MRI | ~ 1% |
| Saved OR Time (3h) | ~ 1% |
| Reduced MER Labor (neuro electrophysiology,intern, support staff…) |
~ 2.5% |
| Saved MER Material (2 boxes of 5, 5 cables, 2 tubes, 1 plaque) |
~ 5% |
| Reduced hospital stays 3 days |
~ 9.5% |
Total cost reduction** Calculation based on a French University Hospital Center with an estimated cost of €40,000 |
~ 19% |
Regulatory Status & Performance Testing
RebrAIn’ services leverages CE Marked and FDA 510(k) cleared SaMD*
The indications for use are:
- Software application intended to aid qualified medical professionals in processing, visualizing, and interpreting anatomical structures from medical images.
- The software can be used to process pre-operative DICOM compatible MR images to generate 3D annotated models of the brain that aid the user in neurosurgical functional planning.
- The annotated MR images can further be used in conjunction with other clinical methods as an aid in localization of the Subthalamic Nuclei (STN) and Ventral Intermediate Nucleus (VIM) regions of interest.
***STN Segmentation
|
clinical
|
clinical
|
| Mean | 0.05 | 0.2 |
| 99% CI | 0-0.11 | 0.08 – 0.32 |
| Max Dist. | 1.6mm | 2mm |
| STN within 2mm | 88/88 100% |
|
| In STN | 85/88 97% |
71/88 81% |
| 100% STN segmentation of RebrAIn within S / G atlas *** Data from the publication of Constantin 2023 |
||
* RebrAIn service leverages CE Marked and FDA 510(k) cleared Software as a Medical Device (SaMD),
RebrAIn OptimMRI and RebrAIn OptimDBS.
Efficiency and Safety
RebrAIn service simplifies surgery planning, significantly shortens operation time, and minimizes health risks and complications.
22 patients underwent surgery under general anesthesia between June 2019 and May 2023(OptiVIM study. 9M/13F, mean age 63 years old, located in FR Lyon and Marseille). |
Preop. |
Postop. |
% |
| The Fahn-Tolosa-Marin scale(Mean ± SD) | 51± 13 | 20 ± 9.5 |
61% CI 95% [53% ; 69%]Giordano et al. JNNP 2020 meta analysis 60% ± 9,7 |
| The mPDQ39(Mean ± SD) | 43± 17 | 22 ± 16 | 49% |
| The SARA (Mean ± SD) |
5 ± 3 | 4 ± 3 | 15% |
The mean distance between the target and the leads (Mean in mm ± SD) : 0.9mm ± 0.7 Min 0mm Max 2.6mm
|
|||
Clinical validation
PROBAVIM Completed
Evaluate DBS targeting of the VIM for essential tremor
64% median improvement in tremor scores; limited by small sample size / Published; retrospective and prospective analysis, 9 patients (FR).
PARKEO Completed
Compare asleep vs. awake STN-DBS for Parkinson’s Disease.
52.3% improvement with asleep surgery; Published; phase 2 trial, 29 patients (FR).
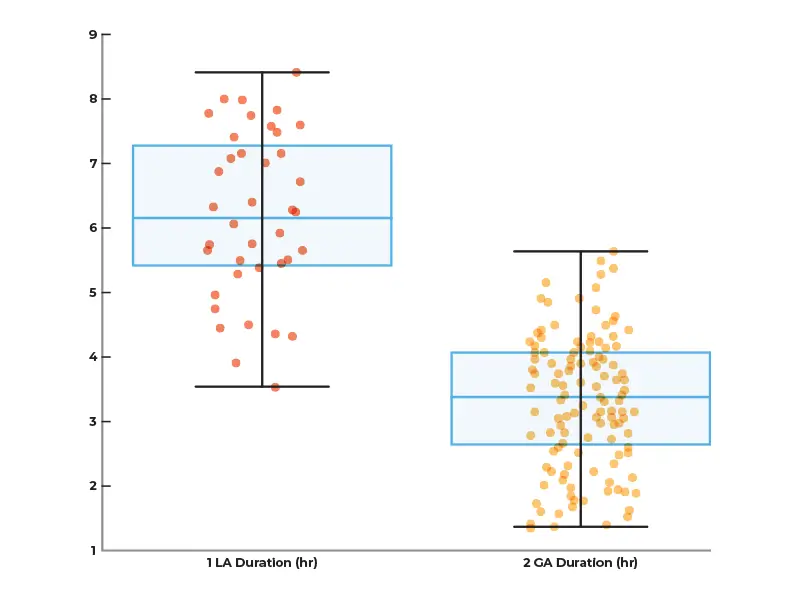
We observed that the average procedure time for surgery with local anesthesia and microelectrode recording (LA/MER) is decreased by 47%+, from 6 hours and 20 minutes to 3 hours and 20 minutes using RebrAln targeting under general anesthesia.
OPTIVIM On-going
- Optimize VIM targeting for essential tremor using novel methods for general anesthesia procedures
- Awaiting results; phase 2 trial, 22 patients (FR)
- (Insight: 61% mean improvement in tremor & 49% improvement in QoL.)
PARKEO 2 On-going
- Evaluate asleep DBS without intraoperative electrophysiology vs. current methods
- Enrolling (phase to end in June 2024); aims for 128 patients, 104 so far (FR)
* RebrAIn service leverages CE Marked and FDA 510(k) cleared Software as a Medical Device (SaMD),
RebrAIn OptimMRI and RebrAIn OptimDBS.